pH, which stands for ‘potential of hydrogen’ or ‘power of hydrogen,’ is a term we encounter in a variety of contexts, from personal care products to gardening, swimming pool maintenance, and industrial processes. The pH scale, which measures the acidity or alkalinity of a substance, is essential for numerous aspects of daily life. This article will explore the meaning, scientific principles, and various applications of pH, helping you understand its importance in both everyday experiences and professional settings.
What Is pH? Full Form of pH
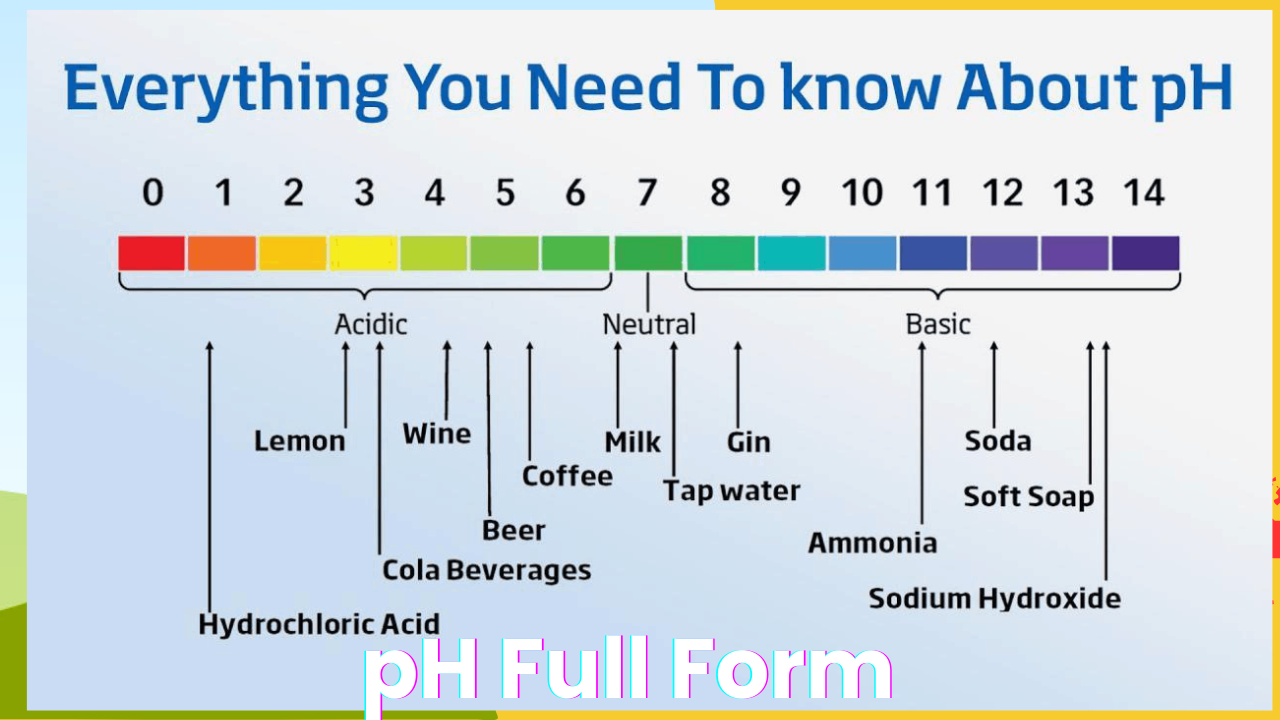
The full form of pH is ‘potential of hydrogen,’ but in practice, it represents the concentration of hydrogen ions (H+) in a given solution. pH plays a crucial role in determining whether a substance is acidic, neutral, or alkaline. On a scale that spans from 0 to 14, a pH value of 7 is considered neutral, meaning it is neither acidic nor alkaline. A pH value below 7 indicates acidity, while a value above 7 signifies alkalinity.
Understanding pH is not just an academic exercise. It has real-world implications in our daily lives and is an essential measurement in many professional fields, from chemistry to medicine to agriculture.
The Science Behind pH: How It Works
At the core of pH measurement is the concept of hydrogen ion concentration. Water molecules naturally dissociate into hydrogen ions (H+) and hydroxide ions (OH-), a process known as self-ionization. In pure water, these ions are present in equal concentrations, giving it a neutral pH of 7.
- Acidity vs. Alkalinity: Substances with a higher concentration of hydrogen ions have a lower pH (acidic). Conversely, substances with a higher concentration of hydroxide ions exhibit a higher pH (alkaline).
- The pH Scale: The scale ranges from 0 to 14. For example:
- 0 to 6 represents acidic substances (strong acid at 0 to weak acid near 6).
- 7 is neutral, which is typically pure water.
- 8 to 14 represents alkaline substances (weak base near 8 to strong base at 14).
Each unit change on the pH scale represents a tenfold difference in acidity or alkalinity, making pH a logarithmic scale. This means that a substance with a pH of 6 is ten times more acidic than one with a pH of 7.
The Role of pH in Everyday Life
While pH is a fundamental scientific concept, it influences many aspects of daily living that might not always be obvious. Whether it’s selecting personal care products or maintaining the right conditions for plants or swimming pools, understanding pH is essential.
1. Personal Care Products
Personal care and beauty products such as shampoos, soaps, and lotions often contain pH information on their labels. Why? Because our skin and hair naturally maintain a certain pH balance for optimal health. For example:
- Skin Health: The skin’s natural pH is slightly acidic, usually ranging from 4.5 to 5.5. Using products with a pH that is too high (alkaline) can disrupt this balance, leading to dry, irritated skin. Conversely, too acidic a product can also cause discomfort.
- Hair Care: Shampoos, conditioners, and hair treatments need to match the pH of hair and scalp to maintain strength and shine. A pH that’s too high can make hair porous and brittle, while too low may cause it to become greasy or dull.
2. Food and Beverages
pH is critical in food safety, preservation, and flavor. Different foods require specific pH levels for proper preparation and preservation:
- Preservation: Many preserved foods, such as pickles and jams, rely on low pH to inhibit bacterial growth and spoilage. Acidic environments prevent microorganisms from thriving, extending shelf life.
- Taste: The pH of ingredients like fruits, vegetables, and dairy can affect their taste, flavor development, and texture. For example, the tartness of citrus fruits comes from their acidic nature.
- Brewing and Fermentation: In the production of beer, wine, and fermented foods like yogurt, maintaining a specific pH is necessary for ensuring the proper fermentation process, which in turn impacts the final product’s taste and consistency.
3. Gardening and Agriculture
Soil pH directly influences plant growth. Plants require different pH levels to absorb nutrients effectively. For instance:
- Acidic Soils (pH < 7): Some plants, such as azaleas and blueberries, thrive in acidic conditions, where nutrient uptake is optimized.
- Alkaline Soils (pH > 7): Other plants, like lilacs and clematis, grow better in alkaline environments.
- Soil Testing: Regular testing of soil pH helps gardeners and farmers adjust the soil composition using amendments like lime (to raise pH) or sulfur (to lower pH), ensuring that crops or flowers grow in the ideal conditions.
4. Swimming Pools
Maintaining the right pH balance in swimming pools is essential for ensuring both safety and comfort:
- Sanitization: Pool water must be slightly alkaline (pH 7.4 to 7.6) for chlorine or other sanitizing agents to work effectively. If the pH is too high or low, the water can become either corrosive or ineffective at disinfecting, leading to potential health hazards.
- Comfort: pH balance also affects swimmer comfort, as water that is too acidic can irritate eyes and skin.
pH in Professional and Industrial Applications
In industrial, medical, and environmental contexts, pH measurements are critical for ensuring safety, product quality, and environmental health.
1. Laboratory Applications
pH is an essential measurement in chemical and biological research:
- Chemical Reactions: Scientists often need to control the pH of a reaction mixture to achieve optimal results. For example, certain enzymes in biological processes function best within a narrow pH range.
- Medical Tests: In medical laboratories, pH is measured in bodily fluids such as blood, urine, and saliva. Abnormal pH levels can indicate underlying health issues like kidney disease, respiratory disorders, or metabolic imbalances.
2. Industrial Applications
Many manufacturing processes require careful pH control:
- Water Treatment: pH is a critical parameter in drinking water treatment. Adjusting the pH ensures the removal of impurities and toxins, making the water safe for consumption.
- Chemical Manufacturing: In the production of pharmaceuticals, textiles, and chemicals, maintaining precise pH levels is crucial for product consistency and quality. pH measurements ensure that the correct reactions occur and prevent unwanted chemical side effects.
3. Environmental Monitoring
Environmental scientists regularly measure the pH of natural water bodies like rivers, lakes, and oceans to monitor ecosystem health:
- Acid Rain: Acid rain, caused by pollutants like sulfur dioxide and nitrogen oxides, lowers the pH of water bodies, negatively affecting aquatic life.
- Ecosystem Health: Changes in the pH of water can indicate pollution or changes in environmental conditions, such as those caused by climate change or industrial waste.
Methods of Measuring pH
There are several ways to measure pH, depending on the application and required accuracy. The choice of method depends on the context, ranging from simple home tests to highly precise measurements in industrial settings.
1. pH Test Strips
pH test strips are a common, easy-to-use method for measuring pH in various applications:
- Home Testing: These strips change color based on the pH of the solution being tested. Users can compare the color change to a standard chart to estimate the pH value.
- Advantages: Simple, inexpensive, and quick. However, they are less accurate than other methods.
2. Digital pH Meters
Digital pH meters provide more accurate and reliable measurements. These devices use a probe to detect the concentration of hydrogen ions in the solution and provide a numerical pH reading.
- Professional Use: Ideal for laboratories, industrial settings, and applications where precision is critical.
- Advanced Features: Many models include temperature compensation to adjust for variations in temperature that can affect pH readings.
3. pH Indicators
pH indicators are chemical substances that change color based on the pH of the solution. Litmus paper is a popular example, but other indicators, like phenolphthalein or bromothymol blue, may also be used in specific contexts.
- Use in Reactions: These indicators are often used in titration experiments or during chemical reactions to visually monitor pH changes.
Conclusion: The Impact of pH in Our Lives and Industries
pH plays a vital role in our daily lives, from maintaining our health to ensuring the quality of the products we use and the environment around us. Whether you are measuring soil pH for gardening, checking pool water quality, or controlling chemical reactions in an industrial process, understanding pH is essential.
In both professional and industrial settings, maintaining the correct pH is crucial for safety, efficiency, and product quality. With a variety of methods available to measure pH, from simple test strips to sophisticated digital meters, there is a solution for every need.
By understanding pH and its applications, you can make better decisions about products, processes, and practices, ensuring optimal outcomes in health, industry, and the environment.
For further guidance on measuring and controlling pH in your field, or for customized water treatment solutions such as commercial RO plants, industrial RO plants, ETP, or STP systems, contact 3D Aqua today. Our experts can provide tailored recommendations and support to optimize your pH management
Contact 3D Aqua:
- Phone: +91-89630-89630
- Email: info@3daqua.in