Water is a vital substance for all forms of life on Earth. Its unique properties contribute significantly to its importance in various biological, chemical, and physical processes. One of the fundamental properties of water is its density, which plays a crucial role in many natural phenomena, industrial applications, and scientific research. This article delves into the density of water, factors influencing it, its significance in different contexts, and its implications for environmental and engineering practices.
Understanding Density

Density is defined as the mass of a substance per unit volume. It is expressed mathematically as:
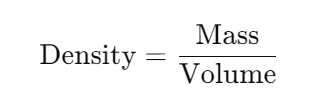
The standard unit of density is grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). For water, the density varies depending on temperature, pressure, and the presence of impurities or solutes.
The Density of Pure Water
At standard temperature and pressure (0°C and 1 atmosphere of pressure), pure water has a density of approximately 1 g/cm³ or 1000 kg/m³. This value serves as a reference point for comparing the density of other substances.
Temperature Influence
One of the most significant factors affecting water’s density is temperature. As the temperature of water changes, so does its density:
- Cold Water: As water cools to about 4°C, its density increases. At this temperature, water reaches its maximum density of approximately 1.000 g/cm³.
- Warm Water: When water is heated above 4°C, it begins to expand, causing its density to decrease. For example, at 100°C, the density of water is about 0.958 g/cm³.
- Frozen Water: Ice, which is water in its solid state, is less dense than liquid water. Ice has a density of about 0.917 g/cm³, which is why it floats on water.
This unusual behavior of water—where it expands upon freezing and reaches its maximum density at 4°C—has profound implications for aquatic ecosystems, as it allows life to thrive in cold climates.
Pressure Influence
While pressure has a minimal effect on the density of liquids compared to gases, it can still play a role in water density. Under high pressure, water’s density increases slightly. However, for most practical purposes, the effect of pressure on water density is negligible in everyday conditions.
Factors Affecting Water Density
In addition to temperature and pressure, other factors can influence the density of water:
1. Impurities and Solutes
The presence of impurities or dissolved substances can significantly alter the density of water:
- Salinity: The addition of salt increases water’s density. For instance, seawater, which has an average salinity of about 35 parts per thousand, has a density of approximately 1.025 g/cm³. This higher density affects buoyancy and ocean circulation.
- Other Solutes: Dissolving sugar, alcohol, or other chemicals in water will also change its density. Each solute has a specific density, and their concentration affects the overall density of the solution.
2. Water Composition
While pure water has a consistent density, natural water sources often contain various dissolved minerals, organic matter, and gases. The specific composition of water—whether from rivers, lakes, or groundwater—can lead to variations in density.
Importance of Water Density
Understanding the density of water is crucial in various fields:
1. Environmental Science
Water density influences the behavior of aquatic ecosystems. For instance, the layering of water in lakes—known as stratification—depends on temperature and density. This stratification affects oxygen levels, nutrient distribution, and the overall health of aquatic habitats.
2. Hydrology and Water Management
Water density plays a vital role in hydrological modeling and water resource management. Accurate knowledge of density helps in predicting water movement in rivers, lakes, and reservoirs, which is essential for flood control, irrigation planning, and water supply management.
3. Engineering Applications
In engineering, water density is a crucial factor in designing various systems, such as:
- Water Treatment Plants: Understanding the density of water is essential for designing processes like sedimentation, filtration, and disinfection. It helps engineers predict how particles will settle in tanks and how to optimize treatment efficiency.
- Hydraulic Systems: In hydraulics, the density of water is necessary for calculating forces, pressures, and flows in systems like pumps, pipes, and spillways.
4. Buoyancy and Hydrodynamics
The density of water directly affects buoyancy, which is the force that allows objects to float. This principle is vital in naval architecture, where the design of ships and submarines must account for water density to ensure stability and safety.
Measurement of Water Density
Measuring the density of water can be accomplished using various methods, including:
1. Hydrometers
A hydrometer is a simple device used to measure the specific gravity (relative density) of liquids. It consists of a sealed glass tube with a scale and a weighted bottom, allowing it to float. The point at which the hydrometer sinks indicates the density of the liquid.
2. Densitometers
Densitometers provide more precise measurements of density using advanced technologies. These instruments often use the principles of oscillation or radiation to measure the density of liquids accurately.
3. Calculations
Density can also be calculated using mass and volume measurements. For example, by measuring the mass of a specific volume of water, one can easily calculate its density.
Practical Applications of Water Density Knowledge
1. Aquatic Ecosystems
Understanding water density helps ecologists study aquatic life. Knowledge of how temperature and salinity affect density can aid in predicting fish migration patterns, breeding habits, and the overall health of marine and freshwater ecosystems.
2. Water Quality Testing
In water quality testing, measuring the density can provide insights into the presence of pollutants or changes in water composition. For example, a sudden change in density may indicate contamination or the introduction of heavy metals or organic matter.
3. Thermal Stratification in Lakes
The concept of thermal stratification in lakes is deeply connected to water density. In summer, warmer water sits on top of colder water, leading to stratification. Understanding this phenomenon is critical for managing fisheries and ensuring water quality.
4. Climate Studies
The density of water is also crucial in climate studies. Oceanographers study water density to understand ocean currents and how they influence global climate patterns. Changes in density due to temperature variations can affect heat distribution in the oceans.
Density of Water at Room Temperature
The density of water can be defined similarly to that of other substances, indicating how much mass exists within a given volume. At room temperature, approximately 20°C, the density of water is about 998.2 kg/m³. When the temperature increases to 25°C, this density decreases slightly to around 997 kg/m³. Importantly, water remains in a liquid state at room temperature, and distilled water maintains this density value.
In contrast, seawater, which contains various salts and minerals, has a higher density of about 1027 kg/m³ at sea level due to these dissolved substances.
Effects of Pressure on Density
Pressure plays a vital role in determining the density of liquids, including water. As pressure increases, the molecules of a substance are forced closer together, which results in a higher density. Conversely, when pressure decreases, the molecules spread apart, leading to a reduction in density. This principle applies to water as well, although the impact is less pronounced compared to gases.
Factors Influencing Water Density
Several factors can affect the density of water:
- Temperature: Water’s density is highly dependent on temperature. It is generally accepted that water has a density of approximately 1 g/cm³ at standard conditions. As water cools, it becomes denser until it reaches around 4°C, where it achieves its maximum density of approximately 1.000 g/cm³. Below this temperature, water begins to expand, resulting in a decrease in density—an unusual property related to its molecular structure.
- Presence of Impurities: The addition of substances such as salts or minerals can alter water’s density. For example, seawater, with its dissolved salts, is denser than pure water.
- Phase of Water: Water has a higher density in its liquid state compared to its solid state (ice). Ice has a density of about 0.917 g/cm³, which is why it floats on liquid water.
Density vs. Temperature
Water does not possess a fixed density; it varies with temperature changes. The following table summarizes the density of water at various temperatures:
| Temperature (°C) | Density (kg/m³) |
|---|---|
| 100 | 958.4 |
| 80 | 971.8 |
| 60 | 983.2 |
| 40 | 992.2 |
| 30 | 995.65 |
| 25 | 997.04 |
| 22 | 997.77 |
| 20 | 998.2 |
| 15 | 999.1 |
| 10 | 999.70 |
| 4 | 998.97 |
| 0 | 999.83 |
| -10 | 998.12 |
| -20 | 993.547 |
| -30 | 983.854 |
Experiment Demonstrating Water Density
To observe the varying densities of liquids, you can conduct a simple experiment using a few common ingredients:
Materials Needed:
- A tall glass or clear mug
- Honey
- Water (dyed with food coloring)
- Coconut oil
Procedure:
- Pour about a quarter cup of honey into the glass.
- Slowly add a quarter cup of colored water over the honey, allowing it to settle without mixing.
- Finally, pour a quarter cup of coconut oil on top of the dyed water.
As you perform this experiment, you’ll observe that the honey sinks to the bottom due to its higher density, while the coconut oil floats on top, demonstrating the concept of density in action.
Comparison of Densities of Various Liquids
Different liquids possess unique densities, as shown in the following table:
| Material | Density (g/cm³) |
|---|---|
| Rubbing Alcohol | 0.79 |
| Lamp Oil | 0.8 |
| Baby Oil | 0.83 |
| Water | 1.0 |
| Milk | 1.03 |
| Liquid Soap | 1.06 |
| Corn Syrup | 1.33 |
| Maple Syrup | 1.37 |
| Honey | 1.42 |
Frequently Asked Questions (FAQs)
Q1. Is the density of water 997 or 1000 kg/m³?
A: At 25°C, the density of water is approximately 997 kg/m³. Pure water at standard conditions is generally considered to have a density of 1000 kg/m³ (or 1 g/cm³).
Q2. What is the density of freshwater?
A: Freshwater has a density of about 1 g/cm³ at 4°C. The addition of salts and other dissolved substances increases the density of seawater to around 1.02 to 1.03 g/cm³.
Q3. How do you calculate the density of water?
A: Density (denoted by the symbol ρ) can be calculated using the formula:
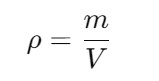
where m is mass and V is volume.
Q4. What is the maximum density of water?
A: The maximum density of water occurs at approximately 4°C. Ice is less dense than liquid water, which is why it floats; freezing causes water’s density to decrease by about 9%.
Q5. Does ice float on water?
A: Yes, ice floats on water because it is about 9% less dense than liquid water. This difference in density allows the lighter ice to float while denser liquid water remains below.
Conclusion
The density of water is a fundamental property that influences various natural and industrial processes. Understanding this property is essential for environmental science, engineering, and numerous other fields. As we continue to explore the intricate relationships between water density, temperature, and solutes, we gain deeper insights into the essential role water plays in our lives.
3D Aqua Water Treatment Company
For those interested in water treatment solutions, 3D Aqua Water Treatment Company is a leading manufacturer of Sewage Treatment Plants (STP), Effluent Treatment Plants (ETP), Reverse Osmosis (RO) systems, Ultrafiltration, and more. With a commitment to quality and sustainability, 3D Aqua offers customized solutions for various industries.
Call now: 89630 89630
Email us: info@3daqua.in
Visit us: www.3daqua.in
Invest in your water management needs with 3D Aqua—where innovation meets quality.